Surgical Options for Early stage RCC
Surgery is almost always utilized for the treatment of patients with renal cell cancer unless patients are too ill to tolerate the procedure. Currently, most physicians agree that the primary cancer should be removed even when patients have evidence that there is small volume spread of the cancer to other sites, if the patient is fit enough.
In patients with stage T1 and T2 renal cell cancer, surgery alone can cure the majority of patients; 75-96% of patients with stage I disease and 63-95% of patients with stage T2 disease are cured with surgery alone. (7)
Surgery can be curative for stage T3 renal cancer depending on the extent of disease, and the percentage of patients with this stage who are cured with surgery alone drops to 38-70%. (7)
Surgical removal of some metastatic cancers can also be curative. Surgery can also relieve symptoms caused by the cancer in patients with stage T3 and T4 renal cell cancer and in those with recurrent disease.
There are several surgical approaches that are utilized, depending on the extent of disease and the condition of the patient.
- Partial Nephrectomy (nephron-sparing surgery):
- open
- laparoscopic
- robotic
- Radical( Total) Nephrectomy:
- open
- laparoscopic
- robotic
- Ablative Therapies:
- Cryotherapy
- Radiofrequency
- Surgery in Advanced Renal Cancer and removal of metastases
Partial Nephrectomy (Nephron-Sparing Surgery)
Removing only the cancer and some surrounding healthy kidney tissue-a procedure called a partial nephrectomy-is now considered the standard of care for the treatment of small renal cancers. The main benefit of this approach is that kidney function is preserved, which is particularly valuable for patients who:
- already have poor kidney function
- have only one kidney
- have lesions in both kidneys (bilateral)
- have an increased tendency to develop cancer in the other kidney (inherited diseases)
The benefits and safety of this approach have been repeatedly demonstrated in the treatment of patients with stage T1a renal cancer, which is defined a cancer that is less than 4 cm in diameter.(8/9)
Partial nephrectomy also appears to be a viable treatment option for patients with stage T1b cancers (which are 4-7 cm in diameter) if an adequate amount of normal tissue surrounding the cancer can be removed.(10) Patients with these slightly larger stage T1b cancers who are treated with partial nephrectomy have been shown to live as long and experience a similar cancer-free duration as patients treated with radical nephrectomy. (11) However, longer follow up aimed at confirming these findings is ongoing.
For those patients with stage T1b cancer that is more centrally located or those with multiple tumours, radical nephrectomy may be a better option.
A detailed and dedicated renal protocol CT scan helps plan the surgery. A renogram might be necessary to elucidate the relevant function of each kidney.
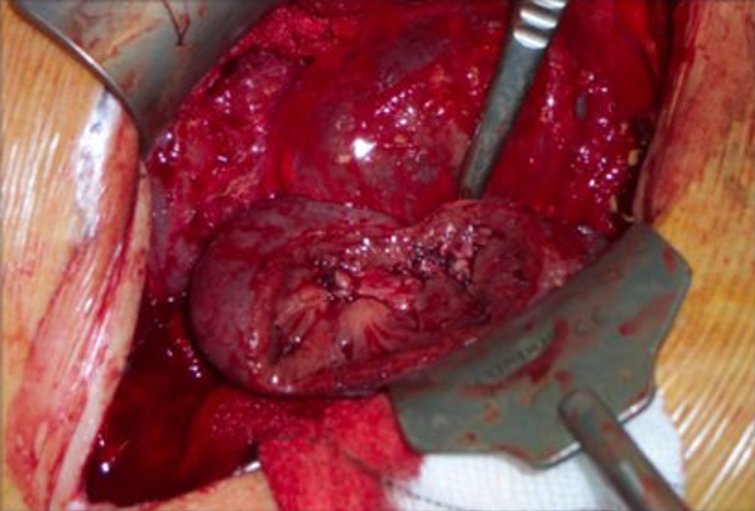
A partial nephrectomy interestingly is a much more complex operation than a radical nephrectomy. This is because it involves removing most of the surrounding fat of the kidney and isolating and clamping of the artery going to the kidney ( renal artery) as well as the vein draining from the kidney ( renal vein) . The purpose of this is to render the kidney free of flowing blood while one cuts into the kidney tissue to excise around the tumour.
Consequently, for a time the kidney does not get the oxygen that the incoming arterial blood usually supplies – a circumstance called ischaemia. Kidneys can tolerate ischaemia for a limited time (30 mins: preferably 20 mins or less) hence putting the surgeon under pressure to do the tumour excision rapidly. In anticipated complex cases (in open surgery) ice slush is sometimes put around the kidney to cool it down allowing the kidney to tolerate a longer ischaemic time (45 minutes).
Complications of partial nephrectomies are similar to that of radical nephrectomies. They carry a more significant risk for intra- and postoperative haemorrhage. Postoperative urinary leaks may occur, and an urinoma (urine collection) may collect which may necessitate drainage. Occasionally a ureteric stent may be inserted to control and slow a urinary leak.
Selected urological surgeons have a high level of experience doing open partial nephrectomies. Patients should avoid the surgeon who only does it occasionally! To do a partial nephrectomy laparoscopically is even more challenging due to the greater difficulty using long rigid instruments. Patients should particularly seek out surgeons with the requisite skills and track record in laparoscopic partial nephrectomy if this option is being considered.
Transperitoneal, retroperitoneal and hand-assisted laparascopic techniques have all been described. In comparative studies, Laparoscopic partial nephrectomy has shown early functional advantages over the open procedure in terms of earlier resumption of diet and shorter hospital stay. (12).
I was one of the early adopters of the Robotic approach to Partial Nephrectomy in the UK.The popularity of the robotic approach has increased considerably as the enhanced dexterity and maneuverability of the “wristed” instruments have made the excision of the tumour and subsequent reconstruction (all done under considerable time pressure whilst the blood vessels are clamped) is easier than pure laparoscopy. Robotic surgery has therefore become the favoured method for minimally invasive approach to Partial Nephrectomy
Radical Nephrectomy
Surgery for some Stage T1 and most StageT2 renal cell cancer typically involves removing the entire affected kidney and the attached adrenal gland, the proximal one-half of the ureter and lymph nodes adjacent to blood vessels entering the middle of the kidney. This is called Radical Nephrectomy.
In some cases, the adrenal gland may not need to be removed. The adrenal glands are complex organs that work with the brain to produce and regulate important hormones, including adrenaline for coping with physical and emotional stress, corticosteroids for suppressing inflammation, and cortisol for controlling the body’s use of fats, proteins, and carbohydrates.
Researchers have reported that patients who underwent nephrectomy but did not have the adrenal gland removed survived as long and were not at any higher risk of postoperative complications than patients who underwent nephrectomy with removal of the adrenal gland. (13)
This operation was traditionally performed as an open procedure via a number of different approaches. Open radical nephrectomy, nowadays is normally reserved for large (i.e. more than 10 cm or more) tumours, or tumours that have invaded the renal vein or further major veins (inferior vena cava). If successfully removed then the likelihood of recurrence in the renal bed has been reported to be 2 to 3% (14).
The Standard approach for a radical nephrectomy nowadays is a minimally invasive approach either Laparoscopic or Robotic Radical Nephrectomy (laparoscopic/robotic radical nephrectomy )
I was one of the pioneers in robotic renal surgery in the UK and feel that the place of robotics is particularly suited to Partial Nephrectomy although a very good approach for Total Nephrectomy. The capability of the fine dissection afforded by the robot is not as important in total nephrectomy as it is an operation where only “removal” of a whole organ takes place, with no reconstruction needed.
Surgery in Advanced Renal Cancer and removal of metastases
Surgery for Stage T4 involves radical nephrectomy if the patient is fit enough. This is followed by systemic therapy. Results from clinical trials have shown that radical nephrectomy might improve survival of patients with metastatic renal cell cancer in selected cases.
For patients with Stage T4 disease whose cancer has spread locally, but not to distant sites in the body, radical nephrectomy may be curative. However, because most patients with Stage T4 renal cell cancer have distant metastases, surgery is typically followed with additional systemic treatment. Surgery is considered a local therapy because it treats cancer in a specific area but does not treat cancer that has spread to other locations in the body.
Some patients can experience long-term cancer-free survival after surgical resection of metastatic cancers. Results of a clinical trial indicate that renal cell cancer that has spread to the lungs in small volume ,can be removed with surgery. Among patients treated with surgery for lung metastases but no evidence of cancer elsewhere in the body, including the kidney, nearly 40% survived five years or more. Patients with only a single site of cancer in the lung experienced the best outcomes; nearly 50% survived five years or more compared to 19% of patients who had more than one site of cancer removed. (15)
An alternative to surgery: It is frequently not possible to perform a radical nephrectomy in older or debilitated patients. In these cases a procedure called arterial embolization is sometimes used to provide relief from pain or bleeding. During arterial embolization small pieces of a special gelatin sponge or other material are injected through a catheter to clog the main renal blood vessel. This procedure shrinks the cancer by depriving it of the oxygen-carrying blood that it needs to survive and grow. Arterial embolization may also be used prior to surgery to make the procedure easier.
Laparoscopic /Robotic Nephrectomy
Laparoscopic or robotic nephrectomy is a well-accepted technique for renal malignancies. Initially, a large tumour size was considered to be a contraindication, but has become less of an issue. The limitation tends to be expertise of the laparoscopic urologist.
It involves inserting rigid tubes (ports) through 3 to 6 tiny (0.5-1cm) holes in the body wall .Long rigid instruments and a camera are placed through these ports thereby enabling one to operate with the help of seeing the image on a TV screen.
The kidney or other tissue is placed in a bag when it has been dislodged from its attachments inside the body and one of the incisions is extended on the skin to pull the bag through.
The advantages for the patients are well described and involve;
- shorter operation time (in skilled hands)
- less blood loss
- rare blood transfusion requirements
- less post operative pain and pain killers
- quicker mobilisation
- shorter hospital stay
- earlier resumption of normal activities.
Approaches can be either transperitoneal (through the front of the abdomen) or retroperitoneal (through the flank). A comparison of the two shows no difference in operating time, cost, and length of stay or postoperative convalescence.
Hand-assisted laparoscopic surgery is also common. This has the advantage of one of the surgeons hands being inside the body and therefore able to retract, dissect and guide the laparoscopic instruments with tactile feedback. Also, it allows a port large enough to remove the kidney whole.It is used generally for larger tumours.
The complication rates for laparoscopic nephrectomy ranges from 8% to 17%, with conversion to an open procedure occurring in up to 4% of cases (16).
Minimally Invasive Ablative Techniques
With ablative techniques the tissue is destroyed in situ rather than be surgically extirpated. The procedures have appeared owing to the demand for minimally invasive techniques and the acceptance of nephron-sparing surgery.
The two most common ablative procedures are cryotherapy and radiofrequency ablation (RFA). Other techniques have been employed such high-focused ultrasound (HIFU), microwave thermotherapy and laser interstitial thermal therapy but are still very much in the development/pilot stage.
Cryotherapy
The first cases od laparoscopic cryotherapy to be performed in the UK were performed by me in December 2004. I have since accumulated one of the largest personal series in Europe and remain an opinion leader in the UK.
What is laparoscopic cryotherapy?
Cryotherapy is a procedure whereby tissue in the body is destroyed by the technique of freezing. The tissues are frozen to an extremely low temperature (-40 degrees Celsius) and cells inevitably undergo complete destruction. There have been many studies shown to prove this.
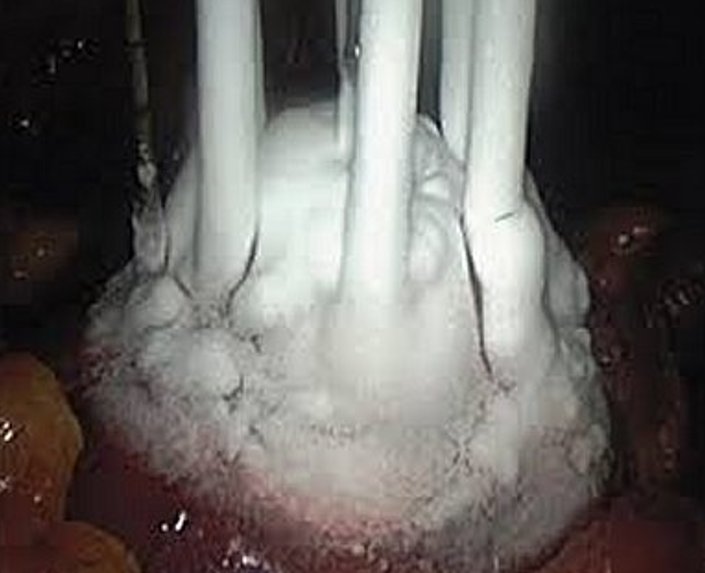
In the context of renal Cryotherapy, the tumour is punctured with needles, which are subsequently frozen with a view to destroying the cancer cells completely. The tumour becomes enveloped in an “ice ball” during the procedure. This results in both immediate and delayed tissue destruction. The tumour is frozen then thawed twice at the same sitting: in so doing the cells are destroyed (necrosis) in the short term and later the frozen tumour becomes scar tissue (fibrosis).
Cryotherapy is a relatively new treatment for early stage renal cancer and although international studies show very promising results there is only medium term follow up. This places the procedure in a category where one cannot comment on the long-term cancer control achieved. Therefore patients should be counseled about this so they are fully informed in making their choice of treatment.
Who is eligible for this treatment?
This method is only applicable to small renal lesions, usually below 3.5cm. It is ideally suited to people who have small renal tumours but who, for a clinical reason, are not able to be subjected to the metabolic demands of a larger open or laparoscopic/robotic operation in order to remove it.
Another group of patients would be those who have a single kidney with either single or multiple tumours. By targeting these lesions with needles one invariably only freezes the tumour cells and spares the surrounding normal renal tissue. It is therefore preferable for such cases as it is a procedure where as much normal renal tissue is spared. It is also ideal in someone who has impaired renal function as the impact of this treatment on renal function is negligible.
A final group in whom it has been shown to be successful is those who have a familial tendency to develop multiple renal tumours from a young age (particularly Von Hippel Lindau syndrome). These patients are likely to require multiple surgical attempts to remove their cancers over a lifetime and it is sensible to spare as much normal renal tissue from the start by using this minimally invasive technique.
How is it done?
The procedure is done as laparoscopically (robotically) or percutaneously.
The laparoscopic/robotic approach is a minimally invasive procedure (keyhole surgery) undertaken by a Urologist with the advantages of minimal post operative pain and earlier return to normal activity. The original technique used was hand-assisted laparoscopy, whereby an incision of a 6-7cm is made through the umbilicus allowing the insertion of the surgeon’s hand to help direct the needles. Subsequently the pure laparoscopic/or robotic approach was adopted. Here, 3 X 1 cm cuts on the skin surface are made through which the instruments are inserted. The procedure takes about 2 hours and done under general anaesthetic. The advantage of the laparoscopic approach is that the tumour can be seen well with unlikely damage to any surrounding organs as the whole procedure is done under vision.
Recently the percutaneous technique has become more popular. A Radiologist performs this by passing the needles directly through the flank under CT imaging guidance. Usually CT scan guidance is used but some centres might use MRI. It is extremely well tolerated by patients as it is the least invasive option, hence its popularity. Not all tumours are suitable though as they might be located in a position where it might be difficult for the needles to reach them percutaneously.
In both techniques, multiple needles are inserted into the tumour and by forcing pressurized Argon gas through the needles one is able to reduce the temperature at the tip of the needle to extremely low temperatures (-150 degrees Celsius). Passing pressurized Helium through them then thaws these needles.
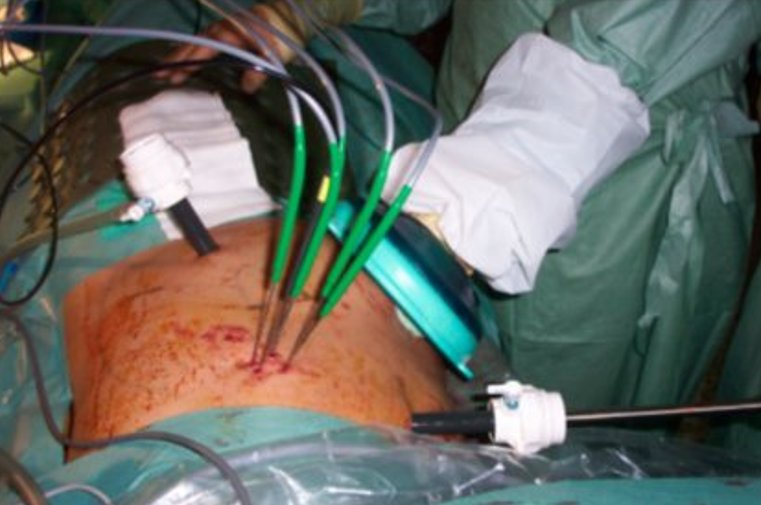
The advantage of the laparoscopic approach is that the tumour can be visualised, the kidney fully mobilised and thus minimise damage to surrounding structures. Monitoring of the ‘ice ball’ produced during the procedure is done under visual and ultrasound guidance.
What to Expect
Most patients go home the following day. Sometimes patients’ recovery can be slower and is influenced by their overall fitness.
Complication rates are low, but one of the worst might be that the patient has bleeding around the kidney post operatively, which might require intervention.( see in the section below regarding expectations of surgery )
A CT scan is performed at 3 months, 6 months and 12 months in the first year and thereafter 6 monthly for 5 years. In the event of tumour recurrence one would have to re-evaluate the best form of management. This might involve a repeat of the same procedure or alternatively a larger undertaking with either partial or total removal of the entire kidney. Performing surgery after Cryotherapy is difficult due to the presence of the scar tissue.
Is the treatment effective?
There have been numerous studies that have shown the efficacy of this method. A large laparoscopic study described the outcomes of 150 patients, of which 56 had more than 3 year follow up. There was 75 % shrinkage of tumour size seen at 3 years and of the entire series there were 2 patients who had recurrence of tumour. The results were best in those patients who have an isolated renal tumour (commonly called sporadic) in one kidney: here there was a 98 % survival rate from renal cancer at 3 years.
In patients who had tumours in both kidneys, the results showed a 3-year survival of 89% .The reason for this is the fact that the tumour treated with Cryotherapy was in some cases obviously being done on a metastasis (spread) rather than a primary tumour. (1)
In a study of 220, biopsy proven, RCC, the local recurrence rate and metastasis free survival were 97.2% and 97.7% at 3 years and 93.9% and 94.4% at 5 years. Major complication rate (Clavien Dindo III) was 4.9%. They concluded 5 year oncological outcomes are competitive with those of surgical resection and at lower complication rate. (2)
A meta- analysis of 3900 patients reached similar conclusions and stated that thermal ablation showed no significant difference in local recurrence or metastases compared with partial nephrectomy. There was lower morbidity and a lesser reduction in eGFR, but with higher all cause mortality and cancer specific mortality. (3)
In one study, among 104 patients treated percutaneously assessed with a mean radiological follow-up of 20.1 months, a single case of unexpected late local recurrence was found. However there were only 62 patients with biopsy proven RCC, which is a very common critique of most studies. (4)
Based on a metaanalysis, when ablating renal tumors, a percutaneous approach was safer than an open or laparoscopic approach and was equally effective. However, more than one procedure was needed to treat the tumour completely. (5)
Generally, of the probe-ablative therapies currently available for renal tumours, Cryotherapy is the most studied and clinically applied treatment. Relatively short-term results are very encouraging but long-term data is needed to compare cancer control with total or partial removal of the kidney. Patients have to be carefully selected: those with small, peripheral, renal lesions are best suited.
Are there limitations?
One of the main problems is that Cryotherapy (and all other ablative treatments like RFA and HIFU) does not generate pathological specimens for the pathologist to study and stage the cancer accurately. Before the procedure a biopsy is taken but this only confirms whether the tumour is a cancer and gives very little more information about the tumour itself. This is in contrast to a partial nephrectomy where the tumour alone or, in total nephrectomy, where the whole kidney is given to the pathologist for analysis.
Another critique is that we have to rely on CT or MRI scans to determine whether there is a good response to Cryotherapy and also whether there is any recurrence of tumour subsequently. This requires long term, meticulous follow up. Patients need to be prepared for and committed to this.
Most studies only have relatively short term follow up. This is particularly relevant in small renal masses as they grow very slowly and oncological outcomes are likely to be good in the short term for almost any treatment approach adopted. Long-term data is needed to determine the real oncological results of Cryotherapy.
Furthermore, Oncological outcomes from previous percutaneous ablation studies are difficult to interpret because of the large number of patients treated previously for RCC as well as the large number of treated renal masses without a pathology- proven diagnosis (6)
References
(1) Gill I S, Remer EM, Hassan WA et al. Renal Cryoablation: outcome at 3 years. J Urol 2005173:1903-7.
(2) David J. Breen, FRCR – Alexander J. King, FRCR – Nirav Patel, FRCR – Richard Lockyer, FRCS – Matthew Hayes, FRCS. Radiology 2018; nn:1–81 – https://doi.org/10.1148/radiol.2018180249
(3) J. Ricardo Rivero, MDa, Jose De La Cerda III, MD, MPHa, Hanzhang Wang, MD, MPHa, Michael A. Liss, MDa,b, Ann M. Farrell, MLSd, Ronald Rodriguez, MD, PhDa,b, Rajeev Suri, MDc, Dharam Kaushik, MD. Partial Nephrectomy versus Thermal Ablation for Clinical Stage T1 Renal Masses: Systematic Review and Meta-Analysis of More than 3,900 Patients. J Vasc Interv Radiol 2017; :1-12 https://doi.org/10.1016/j.jvir.2017.08.013
(4) David J. Breen, Timothy J. Bryant, Ausami Abbas, Beth Shepherd, Neil McGill, Jane A. Anderson†, Richard C. Lockyer, Matthew C. Hayes and Steve L. George.
Percutaneous cryoablation of renal tumours: outcomes from 171 tumours in 147 patients. Onlinelibrary.wiley.com/doi/10.1111/bju12122
(5) Hui GC1, Tuncali K, Tatli S, Morrison PR, Silverman SG. Comparison of percutaneous and surgical approaches to renal tumor ablation: metaanalysis of effectiveness and complication rates. J Vasc Interv Radiol. 2008 Sep;19(9):1311-20. doi: 10.1016/j.jvir.2008.05.014. Epub 2008 Jul 21.
(6) Grant D. Schmit , R. Houston Thompson * , Anil N. Kurup , Adam J. Weisbrod ,Rickey E. Carter † , Matthew R. Callstrom and Thomas D. Atwell. Percutaneous cryoablation of solitary sporadic renal cell carcinomas. Onlinelibrary.wiley.com/doi/10.1111/j.1464-410X.2012.x/pdf
Radiofrequency Ablation
This procedure uses radiofrequency waves and converts them into heat, resulting in thermal (heat) damage to the kidney tissue. The radiofrequency electrodes are introduced either percutaneously or laparoscopically under radiological or visual guidance.
Preliminary results from initial trials were promising but there have been concerns about the reliability of RFA as a monotherapy for RCC. Success of the technique is shown in postoperative CTscans . If the procedure has been successful then the lesion should not enhance with contrast CT. Some studies have shown small areas of still viable cancer in lesions that have been treated with RFA some 12 to 24 months previously.
However, these findings may not be due to the technique, but rather the inability to accurately monitor the lesion during ablation. Improvements are needed to predict and assess the precise margin of ablated tissue (17).
Therapy for advanced Kidney Cancer
Approximately 70 % of patients are potentially curable if they present with localised or locally advanced disease, by nephrectomy alone. Patients with metastatic disease have a median survival of 1 year and 5-year survival of 0-20 %.(18)
Renal cell cancers are typically treated with both local and systemic therapy. Local therapy consists of surgery to remove the entire affected kidney and any surrounding cancer. Systemic therapy is directed at destroying cancer cells throughout the body and may include chemotherapy, targeted therapy, or immunotherapy. The best results are achieved when combining two or more of these approaches.Radiotherapy is applied in selected cases.
Immunotherapy
Immunotherapy works by stimulating the immune system to fight the cancer. There are three general types .The most recent developments have been the use of Checkpoint inhibitors, which have largely replaced the previously used treatments alpha interferon and IL-2 drugs.
Checkpoint inhibitors. The immune system uses a system of “checkpoints” on the surface of cells to tell which ones are normal and which are harmful. Cancer cells can sometimes use checkpoints to pass as healthy cells and hide from the immune system. Checkpoint inhibitors are therefore a newer drug type that turns off checkpoints to help the immune system find cancer cells.
Nivolumab (Opdivo) and pembrolizumab (Keytruda) are two of the more common checkpoint inhibitors that can treat metastatic renal cell cancer.
Interleukin-2 (IL-2) is a man-made version of proteins that the immune system makes called cytokines, which help destroy tumour cells. The medicine activates the immune system to attack the cancer. Previously this was the standard of care for patients with renal cell cancer. It is typically administered in high doses as an inpatient treatment and has historically been associated with severe side effects. However, the safety of high-dose Proleukin has significantly improved over the past decade.
Alpha interferon: Interferon is naturally produced in the body and stimulates the immune system. Alpha interferon is a compound produced in a laboratory that mimics the action of natural interferon and has been shown to stimulate the immune system to recognize and destroy some types of cancer cells.
Unfortunately combination trials of interferon- and interleukin-2 did not show any significant benefit and were shown to be no better than high dose interleukin-2.
Chemotherapy
Chemotherapy has traditionally shown disappointing results in the treatment of RCC. Many trials have explored their potential use but with little avail. Only 10-15% of patients experience an anticancer response to currently available single chemotherapy drugs.
However, combinations of chemotherapeutic agents have given better results. The results are similar to that of cytokine-based immunotherapy and are very much dependant on prognostic factors and individual patients.
Targeted Therapy
A targeted therapy is one that is designed to treat only the cancer cells and minimize damage to normal, healthy cells. The advantages of cancer treatments that “target” cancer cells may include reduced treatment-related side effects and improved outcomes.
These medicines target parts of cancer cells that help them grow and survive. They’re designed to kill cancer without harming healthy cells.
Targeted therapies for renal cell cancer include:
Anti-angiogenesis drugs. Tumours need a blood supply to grow. Angiogenesis is the process that tumours use to make new blood vessels. Anti-angiogenesis therapy cuts off blood vessel growth to “starve” the tumours.
For example, one of these drugs, bevacizumab (Avastin), blocks a protein called VEGF, which helps tumours, grows new blood vessels. It is often taken in conjunction with the immunotherapy drug, interferon alpha.
Tyrosine kinase inhibitors (TKIs) target proteins called tyrosine kinases that help cancer cells and their blood vessels grow. These drugs include:
- Axitinib (Inlyta)
- Cabozantinib (Cabometyx)
- Lenvatinib (Lenvima)
- Pazopanib (Votrient)
- Sorafenib (Nexavar)
- Sunitinib (Sutent)
mTOR inhibitors are medicines that target the mTOR protein, which helps cancer cells grow. They include:
Radiation Therapy
Radiation therapy uses high-energy radiation to kill cancer cells. External beam radiation therapy uses radiation delivered from outside the body that is focused on the cancer. Radiation therapy is sometimes used as the main treatment for kidney cancer for patients whose general health is too poor to undergo surgery. Radiation therapy can also be used to temporarily palliate or ease symptoms of kidney cancer such as pain, bleeding or problems caused by metastasis. Unfortunately, renal cell cancer is not very sensitive to radiation and while the growth of cancer can be slowed, it cannot be entirely eliminated.
Currently, the use of radiation therapy before or after removing the cancer is not routinely recommended because clinical studies have not shown any improvement in patient outcomes.
References
Statistics from Cancer Research UK.
McCredie M, and Stewart JH . risk factors for renal cancer in New South Wales-cigarette smoking, Eur J Cancer 28A(1992) 2050-2054
Prineas RJ, Folsom AR, Zhang ZM.,et al. Nutrition and other risk factors for renal carcinoma in post menopausal women.Epidemiology,8(1997)31-36
Wolk A, Gridley G,Niwa S, Lindblad P,McCredie M, Melemgaard A et al. International renal cell cancer study VII. Role of diet. ,Int J cancer,65(1996)67-63
Ms Laughlin JK, Chow WH, Mandel JS et al. International renal cell cancer study VIII. Role of diuretics , other antihypertensive medications and hypertension. Int J Cancer 63 ( 1995) 216-221.
Vamvakas S , Bruning T , Thomasson B et al.Renal cell cancer correlated with occupational exposure to trichloroethylene.J Cancer Res.Clin .Onc 124(1998) 374-382
Lam JS, Shvarts O, Pantuck AJ. Changing concepts in the surgical management of renal cell carcinoma. European Urology. 2004;45:692-705.
Becker F, Siemer S, Humke U, et al. Elective nephron sparing surgery should become standard treatment for small unilateral renal cell carcinoma: Long-term survival data of 216 patients. European Urology. 2006;49(2):308-13.
Joniau S, Vander Eeckt K, Van Poppel H. The indications for partial nephrectomy in the treatment of renal cell carcinoma. Nature Clinical Practice Urology. 2006;3(4):198-205.
Leibovich BC, Blute ML, Cheville JC, et al. Nephron sparing surgery for appropriately selected renal cell carcinoma between 4 and 7 cm results in outcome similar to radical nephrectomy. Journal of Urology. 2004;171(3):1066-70.
Dash A, Vickers AJ, Schachter LR, et al. Comparison of outcomes in elective partial vs radical nephrectomy for clear cell renal cell carcinoma of 4-7 cm. British Journal of Urology International. 2006;97(5):939-45.
Schiff JD, Palese M, Vaughan ED, Sosa RE, Coll D, Del Pizzo JJ. Laparoscopic versus open partial nephrectomy in consecutive patients: the Cornell experience. BJU Int 2005; 96 (6): 811-14
Siemer S, Lehmann J, Kamradt J, et al. Adrenal metastases in 1635 patients with renal cell carcinoma: outcome and indication for adrenalectomy. Journal of Urology. 2004;171(6 Pt 1):2155-9.
Itano NB. Outcome of isolated renal cell carcinoma fossa recurrence after nephrectomy. J Urol 2000; 164: 322
Friedel G, Hurtgen M, Penzenstadler M, et al. Resection of pulmonary metastases from renal cell carcinoma. Anticancer Research. 1999;19(2C):1593-1596.
Tanagho EA, McAninch JW. Smith’s General Urology 16th Ed. McGraw Hill. New York. Pg 152-156.
Anderson CJ, Havranek EG. Minimally invasive ablative techniques in renal cancer. BJU Int 2004; 93: 707-709
Lam JS, Shvarts O, Leppert JT, Figlin RA, Belldegrun AS. Renal cell carcinoma 2005: new frontiers in staging prognostication and targeted molecular therapy. J Urol 2005; 173 (6): 1853-1862

